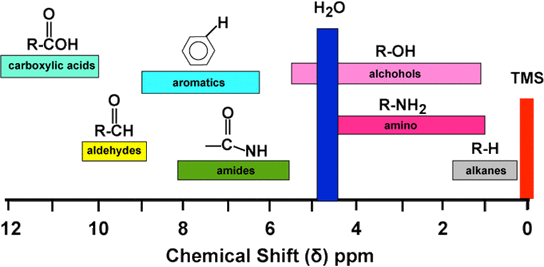
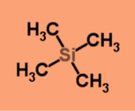
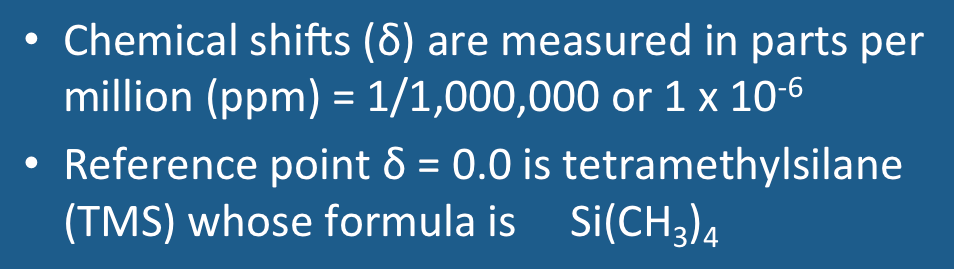 Tetramethylsilane (TMS)
Tetramethylsilane (TMS)
Advanced Discussion (show/hide)»
TMS is not very soluble in water, so for NMR calibration in aqueous solutions, a common alternative reference standard is DSS (4,4-dimethyl-4-silapentane-1-sulfonic acid). This molecule has the structural formula:
(CH3)3Si−CH2−CH2−CH2−SO3−Na+
which is essentially TMS with one of the silane methyl groups replaced by a propyl chain terminated with a sulfonic acid group. Although the spectrum of DSS contains minor peaks from these propyl hydrogens, the dominant peak for calibration still comes from the 9 methyl hydrogens with δ = 0.
Heavier nuclei have relatively larger chemical shifts than 1H. This is primarily due to paramagnetic effects resulting from restriction of free circulation of p and d electrons. Here the (de-)shielding is anisotropic, but averages out for molecules tumbling freely in solution. The relative range of chemical shifts for nuclei commonly used in biomedical NMR are given in the table below along with their typical reference standards:
| Nucleus | Chemical Shift Range | External Reference Standard |
|---|---|---|
| 1H | 15 | TMS or DSS |
| 13C | 250 | TMS |
| 15N | 1000 | CH3NO2 |
| 17O | 600 | D2O |
| 19F | 500 | CCl3F |
| 23Na | 75 | NaBr or NaCl |
| 31P | 300 | H3PO4 |
Meyer LH, Saika A, Gutowsky HS. Electron distribution in molecules. III. The proton magnetic spectra of simple organic groups. J Am Chem Soc 1953; 75:4567-73. (First large published table of NMR chemical shifts of organic molecules)
Reich HJ. Structure determination using spectroscopic methods. Syllabus for Course 605. University of Wisconsin, Madison, 2014. (Contains extensive web-based measurements of hydrogen and carbon chemical shifts). Excellent on-line resource for structural NMR, available at this link.
What is meant by a chemical shift?
How can you predict the chemical shift of hydrogen with a molecule?